The Sophia SATURN study; a randomized, controlled, double-blind clinical trial to study the impact of infant nutrition on early life body fat development.
Study Title
The Sophia SATURN study (Study on infant Adiposity development To Understand the Role of early life Nutrition) is a randomized, controlled, double-blind investigator-initiated clinical trial.
The Sophia Saturn study is registered at the CCMO, with the registration number NL64048.078.18.
Study Objectives
In the Sophia SATURN study, embedded in the Sophia PLUTO study cohort (see below for further details), the impact of Nuturis® on longitudinal infant body fat development is investigated.
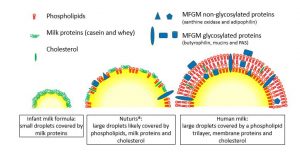 The hypothesis is that infants fed Nuturis® will show a body fat development pattern more similar to that of breastfed infants, compared to standard formula fed infants.
The hypothesis is that infants fed Nuturis® will show a body fat development pattern more similar to that of breastfed infants, compared to standard formula fed infants.
The primary objective of the Sophia SATURN study is to assess the impact of Nuturis® on body fat development. The primary parameter is the change in fat mass index (fat mass/length2) from 6 to 12 months of age. Secondary outcomes include other body composition parameters and growth measurements during the first 2 years of life. In addition, associations of growth and body composition development with maternal characteristics, with metabolic biomarkers and with eating behavior will be explored.
Background
During the first 1,000 days, infants undergo a rapid development in structure and function of their body and its organ systems.
It is known that the development of childhood obesity and metabolic health disorders may originate from a sub-optimal development of metabolic organs during early life. A high maternal pre-pregnancy overweight, prenatal tobacco exposure, Gestational Diabetes, maternal excess gestational weight gain, high infant birth weight, (preterm birth), and unbalanced and/or accelerated infant growth are all known to be associated with adverse metabolic development .1Woo B, Locks L, Cheng E, Blake-Lamb T, Perkins M, Taveras E, Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. Published on 2016;50(6):761-779
Early Nutrition and Body Composition Development
Rapid or unbalanced postnatal growth during the first year of life might cause increased body fat accumulation linked to unfavorable metabolic health effects in later life.2Kerkhof G, Hokken-Koelega A, Rate of neonatal weight gain and effects on adult metabolic health. Nat Rev Endocrinol. 2. Published on 012;8(11):689-692 During the early postnatal period, nutrition plays an important role in supporting infant growth and development.3Leunissen R, Kerkhof G, Stijnen T, Hokken-Koelega A, Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. Published on … Continue reading Breastfed infants have a different growth pattern and body composition development compared to formula-fed infants. After the first months of life, growth rates diverge markedly between breastfed and formula-fed infants leading to a lower weight in breastfed infants at 1 year. Differences in length gain are less pronounced and as a result, breastfed infants have a lower body mass index (BMI, weight/length2) than formula-fed infants at 1 year.4Dewey K, Heinig M, Nommsen L, Peerson J, Lönnerdal B, Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr. Published on 1993;57(2):140-145 A cross-sectional meta-analysis revealed that also differences in body fat development exist, with lower fat mass, fat mass percentage and fat mass index in breastfed infants compared to formula fed infants at 1 year of age.5Gale C, Logan K, Santhakumaran S, Parkinson J, Hyde M, Modi N, Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. … Continue reading However, longitudinal studies describing detailed body composition development of infants in relation with infant nutrition are scarce.
Methodology and intervention
Sophia PLUTO study cohort (CCMO registration NL 39625.078.12)
In the Sophia PLUTO study cohort, longitudinal body fat development is studied in healthy, term born infants from birth until 2 years of age with several consecutive state-of-the-art methods. Infants are recruited from several hospitals in and near Rotterdam. The cohort is led by Professor Anita Hokken-Koelega from the Sophia Children’s Hospital, Erasmus Medical Center, Rotterdam, The Netherlands. The infants have regular outpatient clinic visits up to 2 years at which total body fat (Peapod and/or DXA) and visceral adiposity (Ultrasound) is recorded. Apart from providing reference values for body composition development, the interaction with growth, feeding types and maternal characteristics is investigated.6Breij L. Breij L, Infant body composition and other early life determinants of obesity and adult diseases. Published on April 2016
To learn more about the Sophia Pluto Cohort click here (Erasmus MC website, in Dutch).
Sophia SATURN study (CCMO registration NL64048.078.18)
During early life, adequate nutritional intake is of key importance to support healthy growth and development of infants. Breastfeeding is the gold standard, but unfortunately not always possible. Substitutes should therefore aim to provide nutritional and functional properties and benefits as close as possible to those of human milk. Lipid droplets in infant formulas differ from those in human milk being smaller in size and lacking a phospholipid membrane. Based on the natural fat globule structure of human milk, an innovative infant formula concept Nuturis® was developed with large, phospholipid-coated lipid droplets more similar to those present in human milk.7Gallier S, Vocking K, Post J, et al. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf B Biointerfaces. Published on 2015;136:329-339
Studies supporting Nuturis®
This infant formula concept has shown to alter in vitro lipid digestion kinetics8Van den Braak C, A concept infant formula with large, phospholipid coated droplets demonstrates slow in vitro gastric lipolysis as compared to regular infant formula. JPGN. Published on 2015;60(1):740 and the postprandial lipid response in adult men.9Baumgartner S, van de, Acton D, Mensink R, Infant milk fat droplet size and coating affect postprandial responses in healthy adult men: a proof-of-concept study. Eur J Clin Nutr. Published on … Continue reading In pre-clinical studies, Nuturis® has shown to prevent excessive fat accumulation and adverse metabolic outcomes in nutritional programming models10Oosting A, van V, Kegler D, et al. Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. Br J Nutr. Published on 2014;111(2):215-226 ,11Baars A, Oosting A, Engels E, et al. Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. Br J Nutr. Published on … Continue reading,12Teller IC, Hoyer-Kuhn H, Brönneke H, et al. Complex lipid globules in early-life nutrition improve long-term metabolic phenotype in intra-uterine growth-restricted rats. B. Published on … Continue reading as well as to improve specific cognitive behaviors compared to standard infant formula.13Schipper, A postnatal diet containing phospholipids, processed to yield large, phospholipid-coated lipid droplets, affects specific cognitive behaviors in healthy male mice. J Nutr. Published on … Continue reading Hence, introducing large, phospholipid coated lipid droplets might bring the physiological properties of infant formula closer to that of human milk. In two clinical studies, Nuturis® was demonstrated to be well-tolerated, supporting adequate growth and to be safe for use in healthy term infants; Mercurius study (CCMO registration NL42715.072.12) and Venus study (clinical trial NCT01609634)14Breij L L, An innovative infant formula with large, phospholipid-coated lipid droplets supports an adequate growth in healthy, term infants. Nutrition and Growth Congress. Published on 2016 ,15Shek L, An innovative infant milk formula with large, phospholipid-coated lipid droplets supports an adequate growth and is well-tolerated in healthy, term Asian infants. Nutrition and Growth … Continue reading
For more information on Danone Research & Innovation’s work in OPtimal Growth please see our website.
View References
| 1 | Woo B, Locks L, Cheng E, Blake-Lamb T, Perkins M, Taveras E, Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. Published on 2016;50(6):761-779 |
|---|---|
| 2 | Kerkhof G, Hokken-Koelega A, Rate of neonatal weight gain and effects on adult metabolic health. Nat Rev Endocrinol. 2. Published on 012;8(11):689-692 |
| 3 | Leunissen R, Kerkhof G, Stijnen T, Hokken-Koelega A, Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. Published on 2009;301(21):2234-2242 |
| 4 | Dewey K, Heinig M, Nommsen L, Peerson J, Lönnerdal B, Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr. Published on 1993;57(2):140-145 |
| 5 | Gale C, Logan K, Santhakumaran S, Parkinson J, Hyde M, Modi N, Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. Published on 2012;95(3):656-669 |
| 6 | Breij L. Breij L, Infant body composition and other early life determinants of obesity and adult diseases. Published on April 2016 |
| 7 | Gallier S, Vocking K, Post J, et al. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf B Biointerfaces. Published on 2015;136:329-339 |
| 8 | Van den Braak C, A concept infant formula with large, phospholipid coated droplets demonstrates slow in vitro gastric lipolysis as compared to regular infant formula. JPGN. Published on 2015;60(1):740 |
| 9 | Baumgartner S, van de, Acton D, Mensink R, Infant milk fat droplet size and coating affect postprandial responses in healthy adult men: a proof-of-concept study. Eur J Clin Nutr. Published on 2017;71(9):1108-1113 |
| 10 | Oosting A, van V, Kegler D, et al. Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. Br J Nutr. Published on 2014;111(2):215-226 |
| 11 | Baars A, Oosting A, Engels E, et al. Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. Br J Nutr. Published on 2016;115(11):1930-1937 |
| 12 | Teller IC, Hoyer-Kuhn H, Brönneke H, et al. Complex lipid globules in early-life nutrition improve long-term metabolic phenotype in intra-uterine growth-restricted rats. B. Published on 2018;120(7):763-776. doi 10.1017/s0007114518001988 |
| 13 | Schipper, A postnatal diet containing phospholipids, processed to yield large, phospholipid-coated lipid droplets, affects specific cognitive behaviors in healthy male mice. J Nutr. Published on 2016;146(6):1155-61 |
| 14 | Breij L L, An innovative infant formula with large, phospholipid-coated lipid droplets supports an adequate growth in healthy, term infants. Nutrition and Growth Congress. Published on 2016 |
| 15 | Shek L, An innovative infant milk formula with large, phospholipid-coated lipid droplets supports an adequate growth and is well-tolerated in healthy, term Asian infants. Nutrition and Growth Congress. Published on 2017 |


