A resilient immune system has a positive effect on lifelong health
A resilient immune system has a positive effect on lifelong health
The immune system maintains good health and helps prevent disease by protecting us from harmful substances such bacteria, viruses and toxins, and by removing malignant cells from our system.
A resilient immune system is capable of returning to homeostasis – a healthy state of wellbeing – after an external challenge. An effective working immune system will fight e.g. infections and will down-regulate the immune response once an infection is cleared to prevent harmful responses against the bodies’ own tissue.
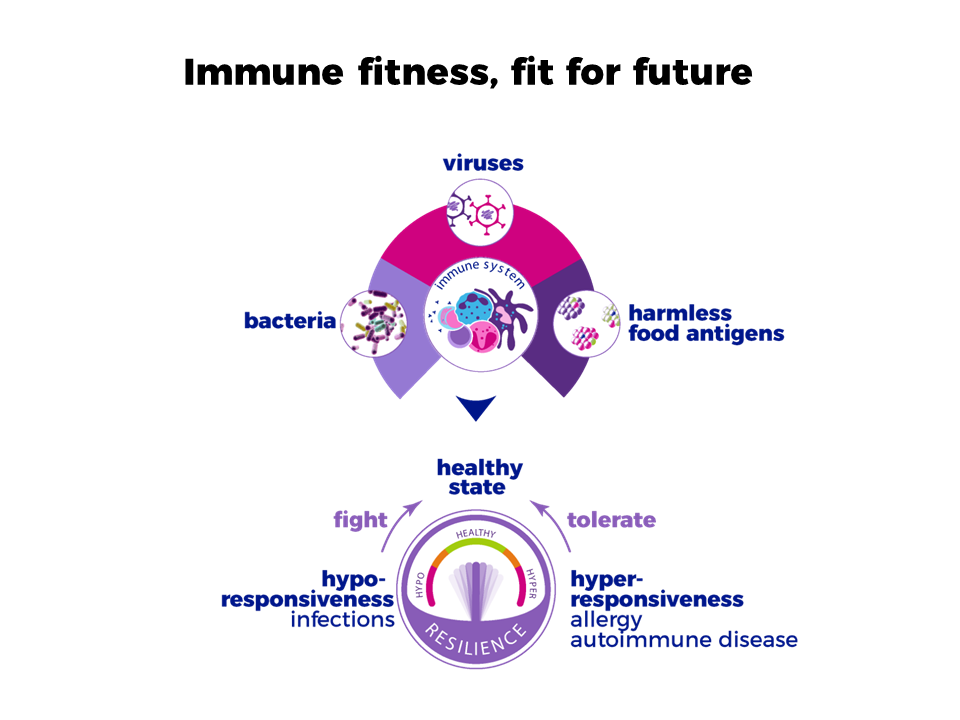
When an individual’s immune system is resilient we also refer to this as immune fitness; having an inbuilt capacity to adapt to challenges by establishing, maintaining and regulating an appropriate immune response.
An appropriate response of the immune system is to eliminate a harmful agent, such as bacteria and viruses, but tolerate harmless ones, such as food. This immune response should be of an optimal strength: not too weak, which will increase the risk of uncontrolled infections, or too strong, potentially resulting in allergy, chronic inflammation, or autoimmune disorders.
The immune system changes throughout life
The immune system is not fully developed at birth, it matures over the first few years of life. A good development of the immune system in early life is associated with an improved health status later in life and vice versa.
An example of this is the ‘Allergic March’, which refers to the fact that suffering from an allergy at young age (such as cow’s milk allergy) predisposes to the development of secondary allergies (e.g. asthma, rhinitis) later on life. Those suffering from allergic disorders now count for 30-40% of the world’s population,1Pawankar R, et al. WAO White Book on Allergy. Published on 2011, Wisconsin contributing to the epidemic in non-communicable diseases (NCDs). Early life is therefore a key phase for training an infant’s immune system.
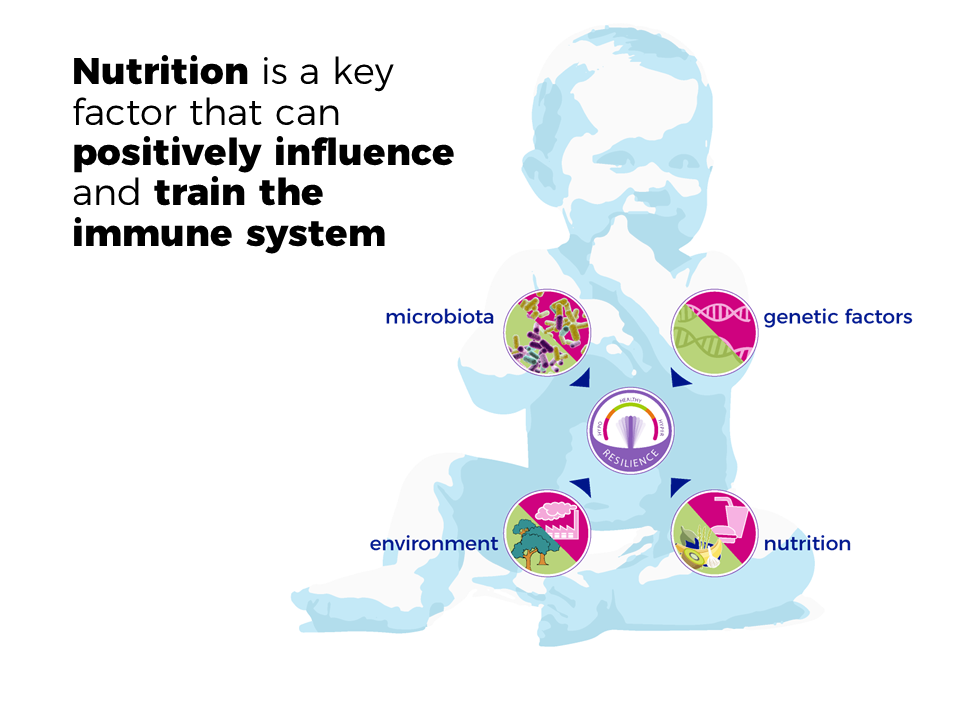
As you grow older, the functioning of your immune system declines. Overall, the immune system responds slower and less effectively, thereby becoming less efficient in its defences and increasing the risk of getting ill. Besides ageing, the immune system can also be impacted by malnutrition, disease or genetic disorders. When the immune system functions less effectively, this leads to a higher risk of a variety of health issues, such as: slower recovery, infections, chronic inflammation, cancer, immobility or autoimmune disorders. It’s therefore important to provide additional support for the immune system throughout life, during the first years to promote development and during later years to maintain optimal function.
Immunity in the gut
Many of our immune cells live in the gastrointestinal tract2West CE, et al. J Allergy Clin Immunol. Published on 2015 Jan;135(1):3-13 along with the 100 trillion gut bacteria that make up the gut microbiota. As the gut is a major entrance for pathogens, toxins and allergens, one of the main roles of the immune system in the gastrointestinal tract is to distinguish between harmless antigens, such as food, and health hazards. The development and preservation of a healthy immune system coincides with the establishment and maintenance of a healthy gut microbiota, and is directly linked to nutrition.3Weizman Z.R, Orel (ed);. Ljubljana Institute for Probiotics and Functional Foods. Published on 2014 ,4Azad MB, et al. Clin Exp Allergy. Published on 2015;45(3):632-43 
An imbalance of gut microbiota is associated with inappropriate immune responses like allergic diseases, (chronic) inflammation and the development of NCDs.5Abrahamsson TR, et al. J Allergy Clin Immunol. Published on 2012;129(2):434-40. e2 Conversely, a healthy gut microbiota is associated with improved health later in life, fewer NCDs, including a reduced risk of allergies and a lower persistence of allergic diseases.,6Pawankar R, et al. WAO White Book on Allergy. Published on 2011, Wisconsin
Read more on: Supporting the immune system through nutrition
View our infographic about the immune system in the gut to learn more.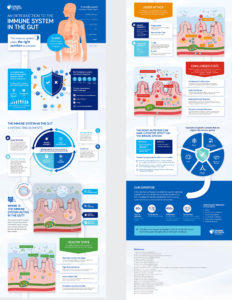
View References
| 1, 6 | Pawankar R, et al. WAO White Book on Allergy. Published on 2011, Wisconsin |
|---|---|
| 2 | West CE, et al. J Allergy Clin Immunol. Published on 2015 Jan;135(1):3-13 |
| 3 | Weizman Z.R, Orel (ed);. Ljubljana Institute for Probiotics and Functional Foods. Published on 2014 |
| 4 | Azad MB, et al. Clin Exp Allergy. Published on 2015;45(3):632-43 |
| 5 | Abrahamsson TR, et al. J Allergy Clin Immunol. Published on 2012;129(2):434-40. e2 |